-
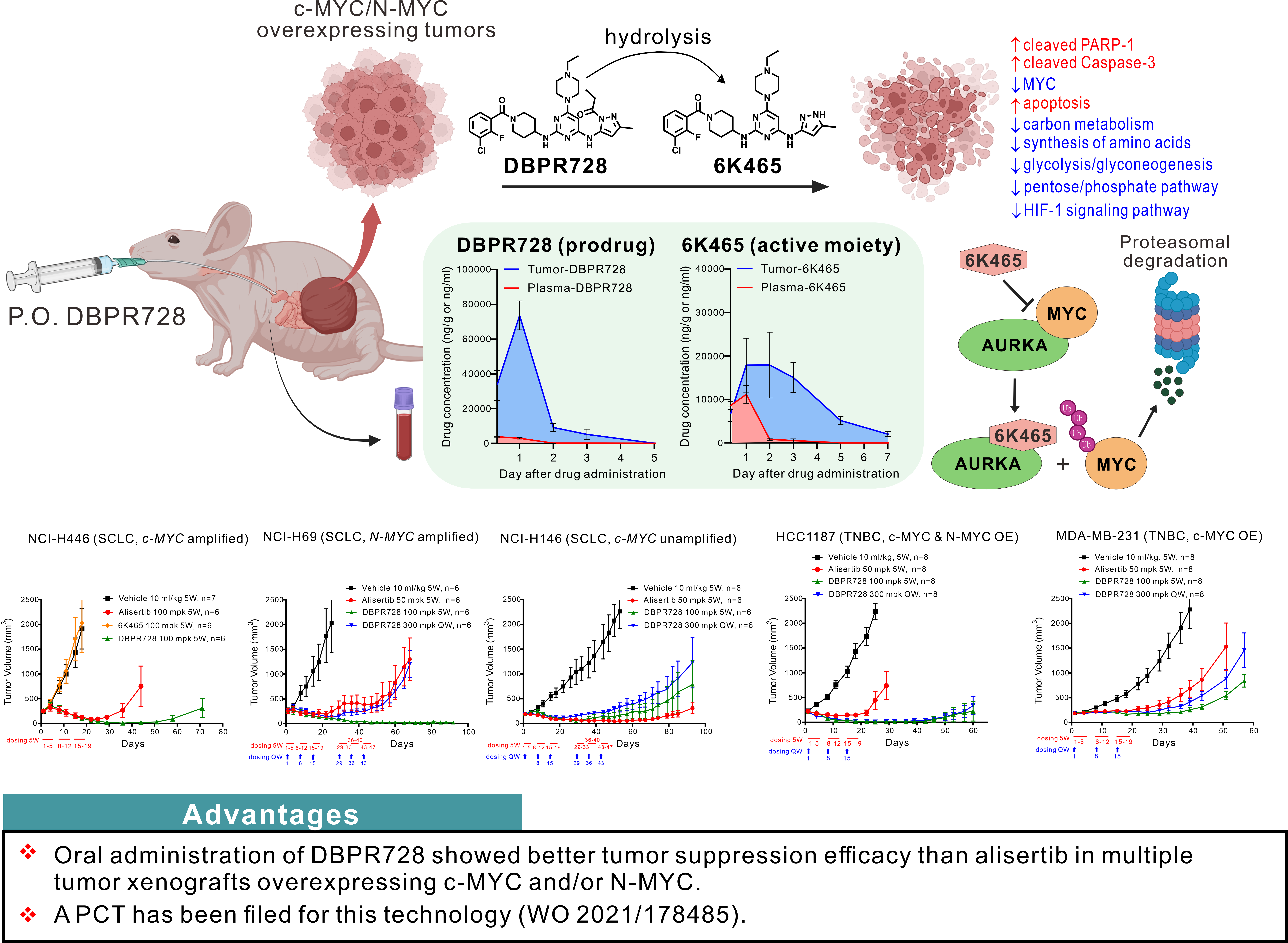
 以MYC致癌基因驅動癌症為標的之激酶抑制劑DBPR728: A Kinase Inhibitor Targeting MYC Driven Cancers國家衛生研究院生技與藥物研究所/IBPR, NHRIInstitute of Biotechnology and Pharmaceutical Research, National Health Research Institutes紀雅惠Ya-Hui Chi發明人:紀雅惠、張竣評、陳炯東、石全、柯屹又領域:癌症標靶新藥適應症:MYC基因增幅或過表現之癌症,以小細胞肺癌和三陰性乳癌為首要標的研發階段:ychi@nhri.edu.tw摘要:DBPR728 is an orally available novel kinase inhibitor drug candidate that promotes the degradation of MYC oncoproteins. DBPR728 has clinical potential for treating cancers with MYC gene amplification or MYC overexpression, providing a clear biomarker for targeted therapy. DBPR728 is a first-in-class innovative drug. Tumors with MYC overexpression can fully utilize glycolysis, allowing cancer cells to metabolize energy in low-oxygen conditions, leading to uncontrolled cell growth and high postoperative recurrence rates, ultimately reducing overall patient survival rates. DBPR728 exhibits high oral absorption and high tumor bioavailability, effectively reducing tumor glycolysis, inducing cancer cell apoptosis, and showing significant tumor regression effects in various MYC high-expression xenograft tumors. This technology has applied for a PCT international patent (WO 2021/178485) and has been granted Taiwan patent I770858.
以MYC致癌基因驅動癌症為標的之激酶抑制劑DBPR728: A Kinase Inhibitor Targeting MYC Driven Cancers國家衛生研究院生技與藥物研究所/IBPR, NHRIInstitute of Biotechnology and Pharmaceutical Research, National Health Research Institutes紀雅惠Ya-Hui Chi發明人:紀雅惠、張竣評、陳炯東、石全、柯屹又領域:癌症標靶新藥適應症:MYC基因增幅或過表現之癌症,以小細胞肺癌和三陰性乳癌為首要標的研發階段:ychi@nhri.edu.tw摘要:DBPR728 is an orally available novel kinase inhibitor drug candidate that promotes the degradation of MYC oncoproteins. DBPR728 has clinical potential for treating cancers with MYC gene amplification or MYC overexpression, providing a clear biomarker for targeted therapy. DBPR728 is a first-in-class innovative drug. Tumors with MYC overexpression can fully utilize glycolysis, allowing cancer cells to metabolize energy in low-oxygen conditions, leading to uncontrolled cell growth and high postoperative recurrence rates, ultimately reducing overall patient survival rates. DBPR728 exhibits high oral absorption and high tumor bioavailability, effectively reducing tumor glycolysis, inducing cancer cell apoptosis, and showing significant tumor regression effects in various MYC high-expression xenograft tumors. This technology has applied for a PCT international patent (WO 2021/178485) and has been granted Taiwan patent I770858.
本技術所揭露之可促進MYC致癌蛋白質降解小分子口服激酶抑制劑DBPR728,在臨床上以帶有MYC致癌基因增幅或具MYC過表現的癌症為疾病標的,具有明確標靶治療生物標記,為first-in-class的新創藥物。帶有MYC基因增幅的腫瘤因為能夠充分利用糖酵解作用,促使癌細胞在缺乏氧氣的情況下進行能量代謝,因此生長速度快,術後復發率也高,造成病患的整體存活率下降。DBPR728具高度口服吸收性以及高腫瘤生體利用率,可有效降低腫瘤進行糖酵解作用,促使癌細胞凋亡,且對多種MYC高表達的異種移植腫瘤具消退效果。該技術已申請PCT國際專利(WO 2021/178485),並獲得台灣專利I770858。
 製備人工視網膜前驅細胞用以治療失明Generation of Retinal Progenitor Cells to Treat Blindness中研院 轉譯中心Biomedical Translation Research Center呂仁Jean Lu發明人:呂仁、蔡榮坤、李岳章、賴培倫、賴倩瑩領域:細胞治療適應症:黃斑部病變、糖尿病引起視網膜病病、夜盲症研發階段:臨床前新藥研究申請準備階段摘要:流行病學調查顯示,失明是一種令人恐懼的疾病,其對人類的影響超越了阿茲海默症和癌症。根據世界衛生組織的數據,全球約有10%的失明病例是由感光細胞退化引起的。我們的研究專注於解決感光細胞退化疾病所帶來的挑戰,市場價值預估將在2030年達到200億美元。本技術利用小分子藥物將人類纖維母細胞誘導為視網膜前驅細胞,轉化效率高達42.8%,僅需5天的時間。這項技術在臨床應用中具有便利性和低成本的優勢。該細胞轉化過程無需基因改造或病毒介入,在動物實驗中已驗證了其治療效果,並且沒有生成腫瘤的風險。與我們的主要競爭對手相比,他們只能拯救黑白視覺,而我們的產品可能能夠恢復黑白和彩色視覺,為患者提供更全面的視覺體驗。我們的技術在醫療市場上具有競爭優勢和發展潛力,特別是在黃斑部病變、糖尿病視網膜病變、和視網膜色素變性(罕見疾病)。我們將與醫療機構和製藥公司建立合作夥伴關係,實現視網膜前驅細胞在細胞治療中的應用,並為感光細胞退化患者提供新的治療選擇。
製備人工視網膜前驅細胞用以治療失明Generation of Retinal Progenitor Cells to Treat Blindness中研院 轉譯中心Biomedical Translation Research Center呂仁Jean Lu發明人:呂仁、蔡榮坤、李岳章、賴培倫、賴倩瑩領域:細胞治療適應症:黃斑部病變、糖尿病引起視網膜病病、夜盲症研發階段:臨床前新藥研究申請準備階段摘要:流行病學調查顯示,失明是一種令人恐懼的疾病,其對人類的影響超越了阿茲海默症和癌症。根據世界衛生組織的數據,全球約有10%的失明病例是由感光細胞退化引起的。我們的研究專注於解決感光細胞退化疾病所帶來的挑戰,市場價值預估將在2030年達到200億美元。本技術利用小分子藥物將人類纖維母細胞誘導為視網膜前驅細胞,轉化效率高達42.8%,僅需5天的時間。這項技術在臨床應用中具有便利性和低成本的優勢。該細胞轉化過程無需基因改造或病毒介入,在動物實驗中已驗證了其治療效果,並且沒有生成腫瘤的風險。與我們的主要競爭對手相比,他們只能拯救黑白視覺,而我們的產品可能能夠恢復黑白和彩色視覺,為患者提供更全面的視覺體驗。我們的技術在醫療市場上具有競爭優勢和發展潛力,特別是在黃斑部病變、糖尿病視網膜病變、和視網膜色素變性(罕見疾病)。我們將與醫療機構和製藥公司建立合作夥伴關係,實現視網膜前驅細胞在細胞治療中的應用,並為感光細胞退化患者提供新的治療選擇。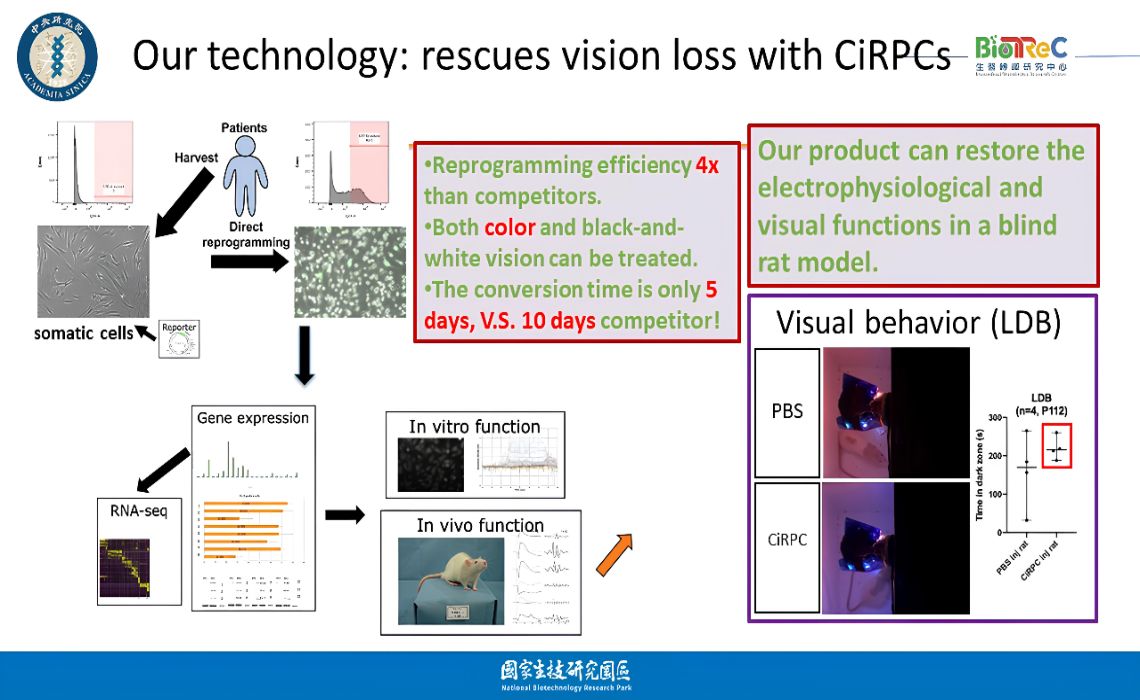
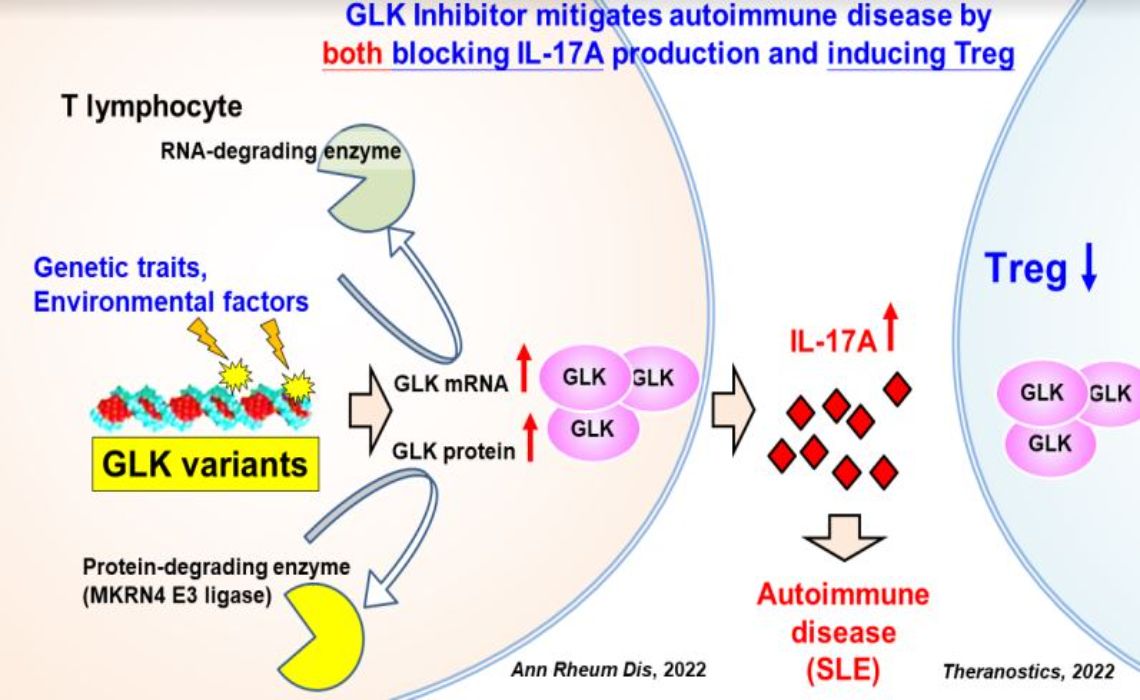
 MAP4K3 (GLK) 為自體免疫疾病、癌症、老化、發炎疾病之新穎治療標靶MAP4K3 (GLK) is a novel therapeutic target for Autoimmune Disease, cancer, aging, and inflammatory disease國家衛生研究院National Health Research Institutes譚澤華特聘研究員, 莊懷佳副研究員Dr. Tse-Hua Tan, Dr. Huai-Chia Chuang發明人:Tse-Hua Tan, Huai-Chia Chuang領域:Immunology, Cancer, Molecular Biology適應症:autoimmune disease, cancer, aging, and inflammatory disease研發階段:Candidates摘要:蛋白激酶MAP4K3 (GLK)刺激T細胞活化。GLK基因剔除小鼠減低T細胞免疫反應,並增長40%壽命。GLK過量表現於自體免疫疾病(如:全身性紅斑狼瘡SLE)、癌症、及COVID-19中,並與疾病嚴重度相關。高達39% SLE病患帶有GLK基因突變。T細胞中,GLK專一地誘發自IL-17A分泌。除此,GLK同時抑制調節性T細胞的分化。因此,抑制GLK可以抑制IL-17A並提高調節性T細胞,達到加乘性抑制自體免疫反應的功效。
MAP4K3 (GLK) 為自體免疫疾病、癌症、老化、發炎疾病之新穎治療標靶MAP4K3 (GLK) is a novel therapeutic target for Autoimmune Disease, cancer, aging, and inflammatory disease國家衛生研究院National Health Research Institutes譚澤華特聘研究員, 莊懷佳副研究員Dr. Tse-Hua Tan, Dr. Huai-Chia Chuang發明人:Tse-Hua Tan, Huai-Chia Chuang領域:Immunology, Cancer, Molecular Biology適應症:autoimmune disease, cancer, aging, and inflammatory disease研發階段:Candidates摘要:蛋白激酶MAP4K3 (GLK)刺激T細胞活化。GLK基因剔除小鼠減低T細胞免疫反應,並增長40%壽命。GLK過量表現於自體免疫疾病(如:全身性紅斑狼瘡SLE)、癌症、及COVID-19中,並與疾病嚴重度相關。高達39% SLE病患帶有GLK基因突變。T細胞中,GLK專一地誘發自IL-17A分泌。除此,GLK同時抑制調節性T細胞的分化。因此,抑制GLK可以抑制IL-17A並提高調節性T細胞,達到加乘性抑制自體免疫反應的功效。
已篩選出GLK小分子抑制劑(verteporfin,IC50= 1.15 nM),可抑制小鼠之自體免疫疾病、癌症、SARS-CoV-2假病毒感染,且抑制SLE病患T淋巴細胞所分泌之IL-17A產生。
GLK為自體免疫疾病、癌症、老化、發炎疾病之新標靶。
GLK (also named MAP4K3) is a critical kinase of T-cell signaling. In T cells, GLK directly interacts with and activates PKCθ, leading to activation of IKK/NF-κB. GLK-deficient mice display impaired T-cell-mediated immune responses, decreased autoimmune phenotypes, and increased 40% life-span. Consistently, the frequencies of GLK-overexpressing T cells are correlated with disease severity of multiple autoimmune diseases. Remarkably, 39% lupus patients harbor GLK germline or somatic variants. GLK signaling in T cells selectively induces IL-17A, which plays critical roles in the pathogenesis of autoimmune diseases. Moreover, GLK also inhibits Treg differentiation. Thus, inhibition of GLK can obliterate autoimmune diseases by both blocking IL-17A production and inducing Treg cells.
Besides autoimmune diseases, GLK is a prognostic biomarker for the recurrence of lung and liver cancers. Furthermore, GLK overexpression in epithelial cells is an important pathogenic factor for COVID-19.
After screening, we identified a small-molecule GLK inhibitor (verteporfin) that inhibited GLK kinase activity (IC50= 1.15 nM), autoimmune diseases in mice, and IL-17A production in human patient T cells. GLK inhibitor also suppresses lung cancer metastasis and SARS-CoV-2 pseudovirus infection in mice. Collectively, GLK is a novel therapeutic target for cancer recurrence, inflammatory diseases, and autoimmune diseases.
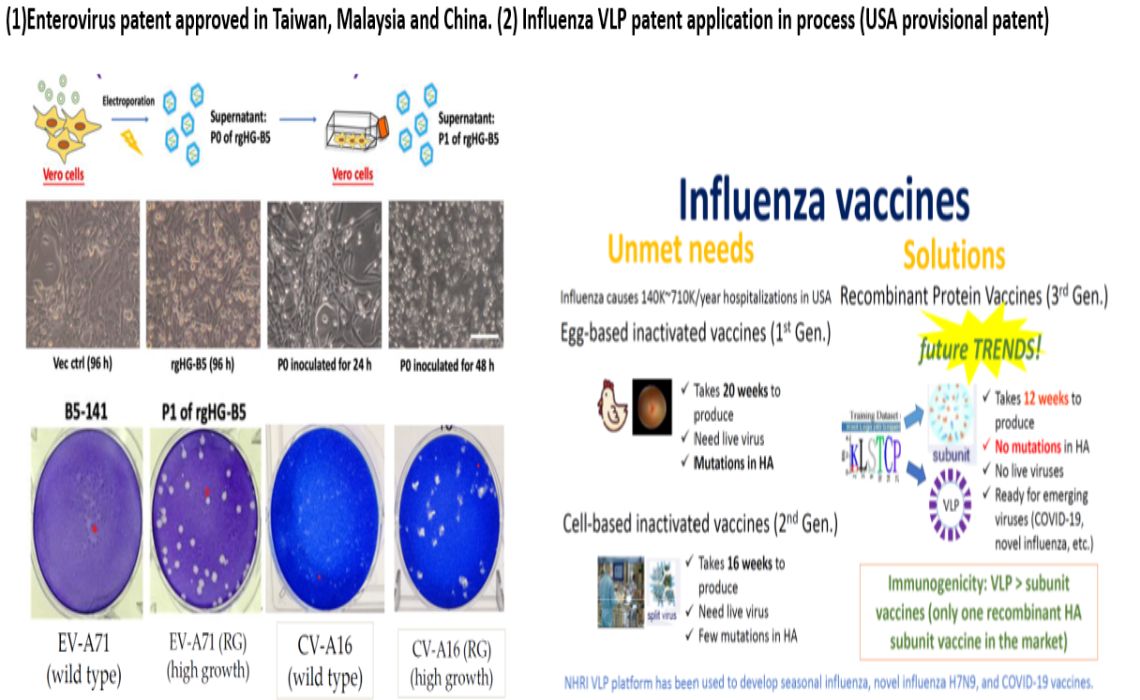
 次世代腸病毒及流感疫苗Next Generation Enterovirus and Influenza Vaccines國衛院疫苗新創-台宇生醫股份有限公司李敏西發明人:李敏西領域:疫苗適應症:雙價腸病毒疫苗的適應症(EV71, CVA16)及季節性流感研發階段:選殖和建立病毒庫、量產測試、臨床前動物試驗摘要:台灣疫苗產業能量有待提升,才能因應新興傳染病的威脅,國衛院李敏西研究員團隊成立新創衍生公司-台宇生醫,致力開發次世代腸病毒及流感疫苗。
次世代腸病毒及流感疫苗Next Generation Enterovirus and Influenza Vaccines國衛院疫苗新創-台宇生醫股份有限公司李敏西發明人:李敏西領域:疫苗適應症:雙價腸病毒疫苗的適應症(EV71, CVA16)及季節性流感研發階段:選殖和建立病毒庫、量產測試、臨床前動物試驗摘要:台灣疫苗產業能量有待提升,才能因應新興傳染病的威脅,國衛院李敏西研究員團隊成立新創衍生公司-台宇生醫,致力開發次世代腸病毒及流感疫苗。
次世代腸病毒疫苗利用高成長腸病毒71型病毒株,顯著提高疫苗生產效率,且對於流行中的B5及C4基因型病毒有良好交叉中和抗體反應,提升了疫苗的生產效率及有效性。
次世代流感疫苗利用昆蟲細胞平台生產類病毒顆粒(VLP),比現行第一代雞胚胎平台和第二代哺乳類細胞培養平台具備更快速及安全的製程,且比次單位疫苗有更佳的免疫力,可大幅降低季節性流感疫苗生產成本,並提高因應新型流感大流行的效率。
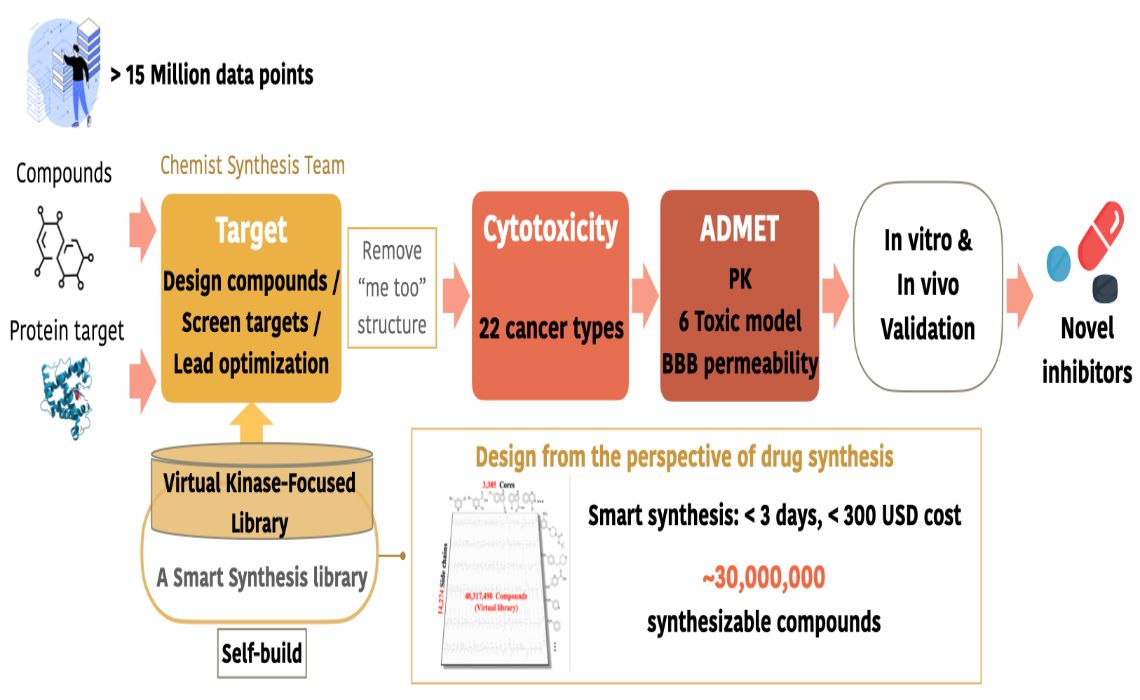
 智慧化創新臨床前新藥研發平台Smart Innovation Platform for Preclinical Drug Development臺北醫學大學Taipei Medical University潘秀玲Pan, Shiow Lin發明人:潘秀玲、許凱程領域:新藥研發適應症:癌症與神經退化性疾病研發階段:候選藥物/醫材雛型試製造 Pilot production of candidate drug/prototype摘要:智慧化創新臨床前新藥研發平台為小分子新藥研發者解決研發高失敗率問題,我們提供AI研發服務與早期新藥產品。我們價值在於快速又有效:新穎結構的可合成率80%、降低合成成本90%、細胞實驗驗證有效率60%(IC50<10µM)。預計可縮減臨床前研發時程至2年。相比於其他AI公司,我們以化學、生物、AI專家所組成的團隊更解決了AI設計的新結構所面臨到的合成問題。目前本團隊已應用此平台於4年內開發出29個高潛力新穎小分子抑制劑研發專案(IC50<10nM),且多數在臨床上無競爭產品,並已服務18個國內外客戶。
智慧化創新臨床前新藥研發平台Smart Innovation Platform for Preclinical Drug Development臺北醫學大學Taipei Medical University潘秀玲Pan, Shiow Lin發明人:潘秀玲、許凱程領域:新藥研發適應症:癌症與神經退化性疾病研發階段:候選藥物/醫材雛型試製造 Pilot production of candidate drug/prototype摘要:智慧化創新臨床前新藥研發平台為小分子新藥研發者解決研發高失敗率問題,我們提供AI研發服務與早期新藥產品。我們價值在於快速又有效:新穎結構的可合成率80%、降低合成成本90%、細胞實驗驗證有效率60%(IC50<10µM)。預計可縮減臨床前研發時程至2年。相比於其他AI公司,我們以化學、生物、AI專家所組成的團隊更解決了AI設計的新結構所面臨到的合成問題。目前本團隊已應用此平台於4年內開發出29個高潛力新穎小分子抑制劑研發專案(IC50<10nM),且多數在臨床上無競爭產品,並已服務18個國內外客戶。
The smart innovation platform for preclinical drug development addresses the high failure rate problem for small molecule new drug developers. We provide AI research and development services as well as early-stage new drug products. Our value lies in speed and efficiency: an 80% synthetic rate for novel structures, a 90% reduction in synthesis costs, and a 60% validation efficiency in cell experiments (IC50 < 10µM). It is expected to reduce preclinical development timelines to within two years. Compared to other AI companies, our team, composed of experts in chemistry, biology, and AI, effectively addresses the synthesis challenges posed by AI-designed new structures. In the past four years, our team has used this platform to develop 29 high-potential novel small molecule inhibitor projects, most of which have no competitive products in the clinical stage, and have served 18 domestic and international clients.
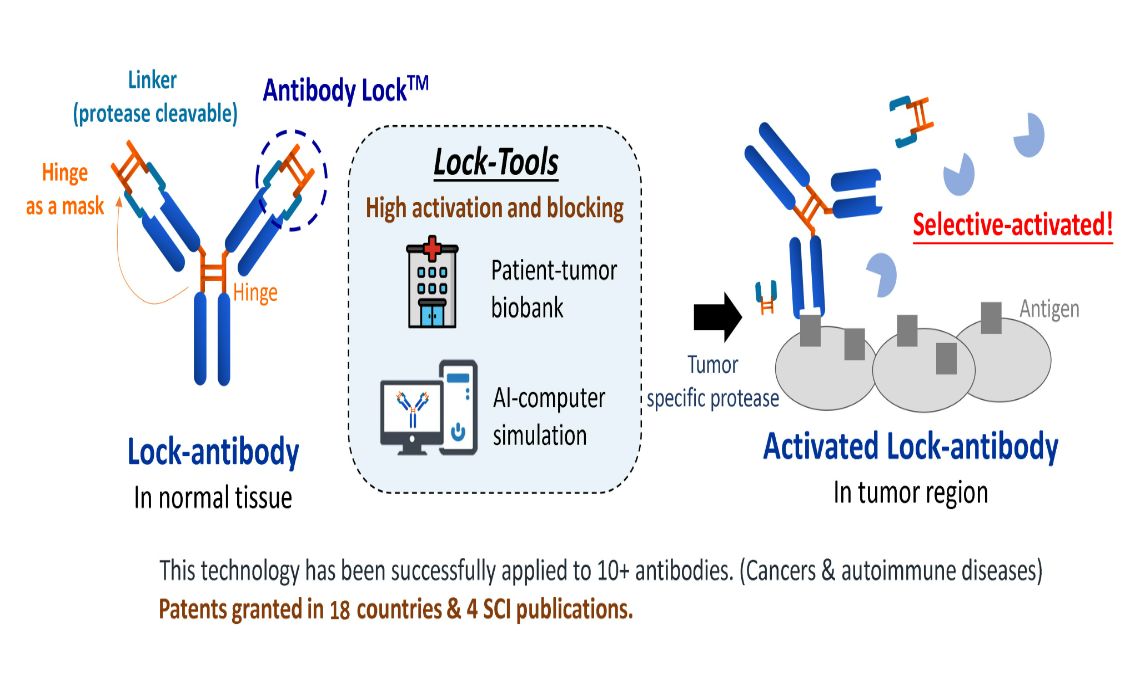
 萬能抗體鎖Universal Antibody Lock碩準生技股份有限公司PrecisemAb Biotech Co., Ltd.呂韻綺Yun-chi Lu發明人:鄭添祿,莊智弘,柯秀芬,呂韻綺領域:新藥開發適應症:頭頸癌,直腸癌,肺癌研發階段:臨床前摘要:「萬能抗體鎖技術」透過創新的屏蔽結構,有效改善現今抗體藥物選擇性不足的問題,提升抗體對疾病區域的選擇性,大幅降低藥物副作用,不僅為抗體藥物安全性帶來革命性突破,也大大提高病人的生活品質。該技術可應用於單克隆抗體藥物、抗體藥物複合體(ADC)、雙功能抗體等領域。PSM101 使用碩準獨創的抗體鎖技術改造已上市的 anti-EGFR 抗體 Erbitux®,形成 Lock-EGFR Ab。臨床發現,使用 EGFR 小分子或單克隆抗體治療癌症的患者,其治療效果與皮膚毒性程度相關。治療效果越好,皮膚毒性越嚴重,導致患者需要降低劑量或中止治療。為解決此問題,碩準授權自高醫大鄭添祿教授團隊的抗體鎖技術平台,使 PSM101 僅在腫瘤部位被腫瘤特異性蛋白酶活化,專一性結合腫瘤,發揮毒殺和生長抑制作用,同時減少對正常組織的非特異性結合。此平台能通過基因工程加裝抗體鎖,減少對正常細胞的傷害,並在疾病區域通過蛋白酶解鎖抗體功能,達到精準治療效果。
萬能抗體鎖Universal Antibody Lock碩準生技股份有限公司PrecisemAb Biotech Co., Ltd.呂韻綺Yun-chi Lu發明人:鄭添祿,莊智弘,柯秀芬,呂韻綺領域:新藥開發適應症:頭頸癌,直腸癌,肺癌研發階段:臨床前摘要:「萬能抗體鎖技術」透過創新的屏蔽結構,有效改善現今抗體藥物選擇性不足的問題,提升抗體對疾病區域的選擇性,大幅降低藥物副作用,不僅為抗體藥物安全性帶來革命性突破,也大大提高病人的生活品質。該技術可應用於單克隆抗體藥物、抗體藥物複合體(ADC)、雙功能抗體等領域。PSM101 使用碩準獨創的抗體鎖技術改造已上市的 anti-EGFR 抗體 Erbitux®,形成 Lock-EGFR Ab。臨床發現,使用 EGFR 小分子或單克隆抗體治療癌症的患者,其治療效果與皮膚毒性程度相關。治療效果越好,皮膚毒性越嚴重,導致患者需要降低劑量或中止治療。為解決此問題,碩準授權自高醫大鄭添祿教授團隊的抗體鎖技術平台,使 PSM101 僅在腫瘤部位被腫瘤特異性蛋白酶活化,專一性結合腫瘤,發揮毒殺和生長抑制作用,同時減少對正常組織的非特異性結合。此平台能通過基因工程加裝抗體鎖,減少對正常細胞的傷害,並在疾病區域通過蛋白酶解鎖抗體功能,達到精準治療效果。
 靶向血小板αIIbβ3組合蛋白之低出血風險溶栓劑成大生技醫藥研發中心張耀宗發明人:莊偉哲、黃德富、郭育汝領域:適應症:NSTEMI, Unstable angina, PCI, Ischaemic stroke研發階段:先導藥物最佳化 Lead drug optimization摘要:現行溶栓治療或介入性導管治療需搭配抗血小板凝集藥物,以避免血管再窄化或再復發的情形。然而,抗血小板凝集藥物均有出血與血小板低下症副作用,亦是待被解決之問題。αIIbβ3-specific拮抗劑 (RR) 除了具有更高抑制血小板凝集的活性外,還具有不影響血小板黏附與出血之優點。出血結果顯示RR蛋白展現與控制組相似的出血時間,與現有藥物abciximab造成的出血時間相比減少了10倍以上。再者,實驗證據顯示RR蛋白可用於肌肉注射,在施打17小時後仍保有50%的抑制血小板凝集活性,意指RR在使用上更有便利性。
靶向血小板αIIbβ3組合蛋白之低出血風險溶栓劑成大生技醫藥研發中心張耀宗發明人:莊偉哲、黃德富、郭育汝領域:適應症:NSTEMI, Unstable angina, PCI, Ischaemic stroke研發階段:先導藥物最佳化 Lead drug optimization摘要:現行溶栓治療或介入性導管治療需搭配抗血小板凝集藥物,以避免血管再窄化或再復發的情形。然而,抗血小板凝集藥物均有出血與血小板低下症副作用,亦是待被解決之問題。αIIbβ3-specific拮抗劑 (RR) 除了具有更高抑制血小板凝集的活性外,還具有不影響血小板黏附與出血之優點。出血結果顯示RR蛋白展現與控制組相似的出血時間,與現有藥物abciximab造成的出血時間相比減少了10倍以上。再者,實驗證據顯示RR蛋白可用於肌肉注射,在施打17小時後仍保有50%的抑制血小板凝集活性,意指RR在使用上更有便利性。
The current fibrinolytic therapy or percutaneous coronary intervention (PCI) needs to be combined with anti-platelet drugs in a case of subsequent restenosis or recurrent thrombosis. However, antiplatelet coagulant drugs are associated with bleeding and thrombocytopenia side effects that need to be addressed. αIIbβ3-specific antagonists (RR) have higher antiplatelet activity and do not affect platelet adhesion and bleeding. The bleeding model showed that Tmu RR mutant exhibited similar bleeding time as control and had >10-fold decrease in bleeding time as compared with the commercial abciximab. We also found that RR exhibited the 50% activity to inhibit platelet aggregation of 17 hrs using intramuscular injection, suggesting that RR is more convenient in use.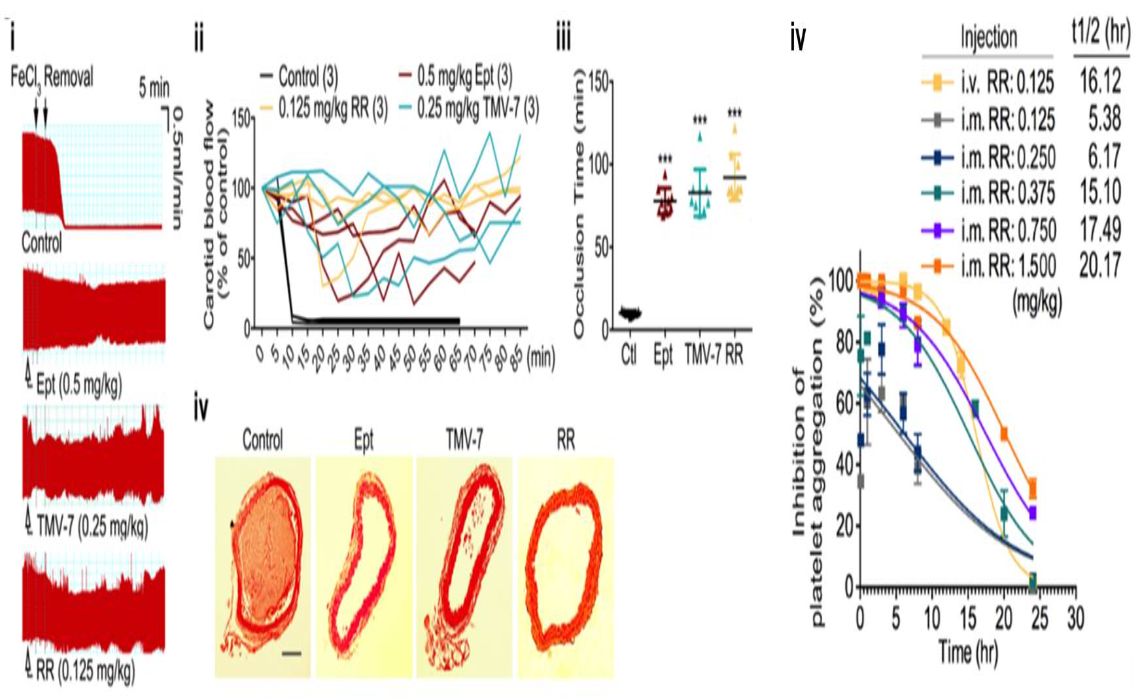
 NuPlus: 以LNP包覆之RNA類DDB2抑制劑做為化療增敏劑NuPlus: RNA-Based DDB2 Inhibitor in LNP as a Chemosensitizer中國醫藥大學China Medical University黃偉謙/何宥豪/周心喬Wei-Chien Huang / Yu-Hao He/Hsin-Chiao Chou發明人:黃偉謙、何宥豪、魏雅鈴、姚俊旭、周德陽領域:Oncology適應症:乳癌、肺癌、肝癌、膽囊癌、胃癌研發階段:non-GLP validation摘要:化療是所有乳癌亞型的標準治療,但整體治療的反應率僅為50%。NuPlus為解決臨床需求,旨在成為第一個以核酸抑制DDB2的化療增敏劑,透過增加化療敏感性、減少藥物劑量及副作用、有效抑制腫瘤,提供更好的治療選擇並改善患者的生活品質。NuPlus以脂質奈米顆粒(LNP)作為藥物遞送的策略。下一階段,NuPlus將規劃與GMP製藥公司合作,進行包覆系統的合作與授權許可,以實現雙方的快速盈利。在完成PoC階段後,NuPlus即將進入關鍵的藥物開發階段。我們在台灣萌芽計劃經費補助下,將進行non-GLP的藥理效力驗證、初步安全性評估和基本藥代動力學測試,為進入臨床前試驗階段做準備。
NuPlus: 以LNP包覆之RNA類DDB2抑制劑做為化療增敏劑NuPlus: RNA-Based DDB2 Inhibitor in LNP as a Chemosensitizer中國醫藥大學China Medical University黃偉謙/何宥豪/周心喬Wei-Chien Huang / Yu-Hao He/Hsin-Chiao Chou發明人:黃偉謙、何宥豪、魏雅鈴、姚俊旭、周德陽領域:Oncology適應症:乳癌、肺癌、肝癌、膽囊癌、胃癌研發階段:non-GLP validation摘要:化療是所有乳癌亞型的標準治療,但整體治療的反應率僅為50%。NuPlus為解決臨床需求,旨在成為第一個以核酸抑制DDB2的化療增敏劑,透過增加化療敏感性、減少藥物劑量及副作用、有效抑制腫瘤,提供更好的治療選擇並改善患者的生活品質。NuPlus以脂質奈米顆粒(LNP)作為藥物遞送的策略。下一階段,NuPlus將規劃與GMP製藥公司合作,進行包覆系統的合作與授權許可,以實現雙方的快速盈利。在完成PoC階段後,NuPlus即將進入關鍵的藥物開發階段。我們在台灣萌芽計劃經費補助下,將進行non-GLP的藥理效力驗證、初步安全性評估和基本藥代動力學測試,為進入臨床前試驗階段做準備。
Chemotherapy is the standard treatment for all subtypes of breast cancer, but its overall response rate is only 50%. NuPlus addresses this clinical need by becoming the first nucleic acid-based chemosensitizer targeting DDB2. By enhancing chemotherapy sensitivity, reducing drug dosage and side effects, and effectively inhibiting tumor growth, NuPlus aims to provide better treatment options and improve patients' quality of life. Utilizing lipid nanoparticles (LNP) for drug delivery, the next phase involves collaborating with GMP pharmaceutical companies for system encapsulation and licensing, ensuring mutual rapid profitability. Having completed the PoC stage, NuPlus is set to enter critical drug development. With funding from the Taiwan Germination Program, we will conduct non-GLP pharmacodynamic validation, preliminary safety assessment, and basic pharmacokinetic testing, preparing for the preclinical trial phase.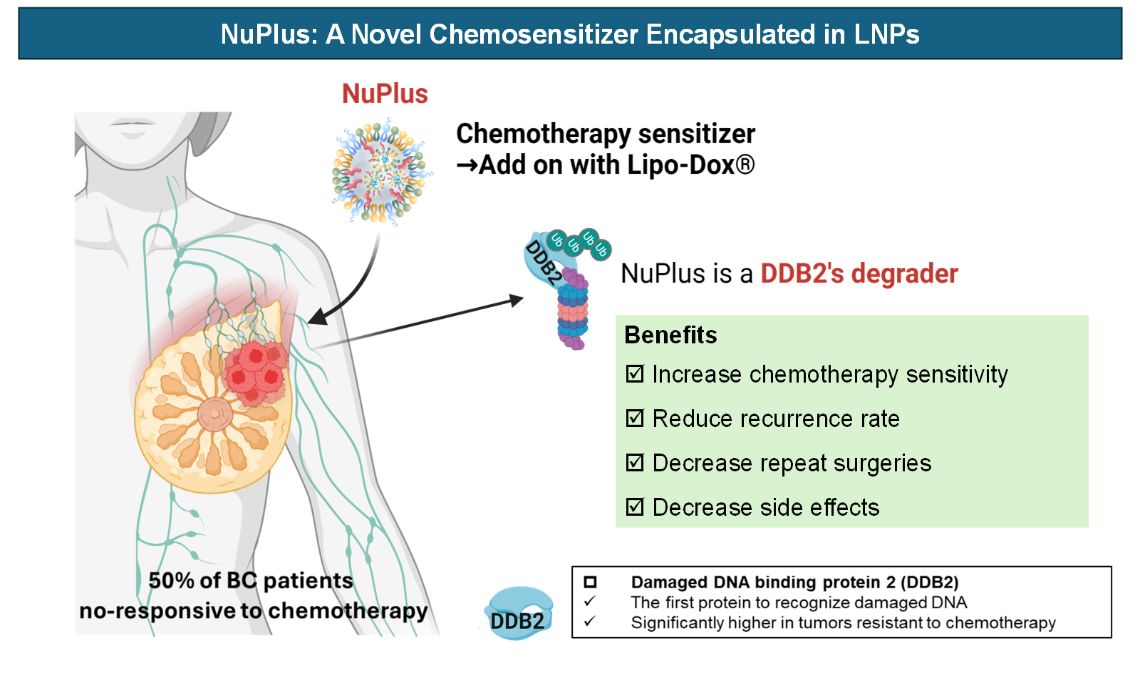
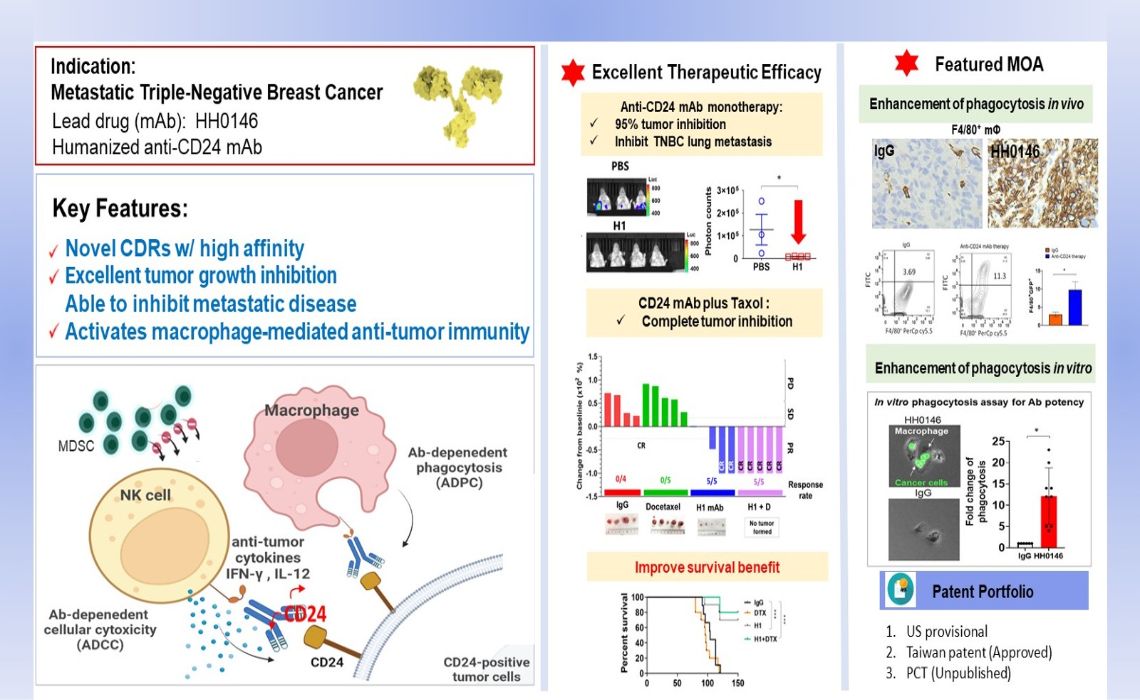
 治療性人源化CD24單株抗體作為新一代的免疫標靶療法Therapeutic humanized anti-CD24 mAb as a next-generation immune targeted therapy中國醫藥大學Chinal Medical University詹世萱Shih-Hsuan Chan發明人:詹世萱, 王陸海, 徐祖安, 洪慧貞領域:單株抗體新藥適應症:轉移性三陰性乳癌研發階段:Pre-clinical摘要:Triple-negative breast cancer (TNBC) is the subtype of breast cancer that is most prone to recurrence and distant metastasis. The limited availability of targeted therapies makes it a significant challenge in clinical breast cancer treatment, resulting in an unmet medical need for metastatic TNBC. In 2019, our research team was the first to propose the potential of anti-CD24 therapy as a new treatment for metastatic TNBC. Recently, CD24 has been confirmed as an immune checkpoint in macrophages, opening new opportunities for the next generation of immune checkpoint therapies. Our team has developed the therapeutic humanized anti-CD24 antibody HH0146, which is at the forefront of domestic patent technology for humanized anti-CD24 antibody targeting tumors. HH0146 can specifically activate the excellent phagocytosis of macrophages while also activating natural killer cells to kill tumors, thereby modulating the tumor immune microenvironment and achieving outstanding anti-tumor efficacy. The HH0146 antibody has obtained a Taiwanese invention patent and has completed international patent application filings (PCT/US24/13014: unpublished).
治療性人源化CD24單株抗體作為新一代的免疫標靶療法Therapeutic humanized anti-CD24 mAb as a next-generation immune targeted therapy中國醫藥大學Chinal Medical University詹世萱Shih-Hsuan Chan發明人:詹世萱, 王陸海, 徐祖安, 洪慧貞領域:單株抗體新藥適應症:轉移性三陰性乳癌研發階段:Pre-clinical摘要:Triple-negative breast cancer (TNBC) is the subtype of breast cancer that is most prone to recurrence and distant metastasis. The limited availability of targeted therapies makes it a significant challenge in clinical breast cancer treatment, resulting in an unmet medical need for metastatic TNBC. In 2019, our research team was the first to propose the potential of anti-CD24 therapy as a new treatment for metastatic TNBC. Recently, CD24 has been confirmed as an immune checkpoint in macrophages, opening new opportunities for the next generation of immune checkpoint therapies. Our team has developed the therapeutic humanized anti-CD24 antibody HH0146, which is at the forefront of domestic patent technology for humanized anti-CD24 antibody targeting tumors. HH0146 can specifically activate the excellent phagocytosis of macrophages while also activating natural killer cells to kill tumors, thereby modulating the tumor immune microenvironment and achieving outstanding anti-tumor efficacy. The HH0146 antibody has obtained a Taiwanese invention patent and has completed international patent application filings (PCT/US24/13014: unpublished).
三陰性乳癌是乳癌中最容易復發和遠端轉移的亞型,由於適用的標靶藥物有限,使得其成為臨床乳癌治療的一大挑戰,因此轉移性三陰性乳癌仍是未被滿足的醫療需求。研發團隊在2019年率先提出抗CD24療法有潛力作為治療轉移性三陰性乳癌的新療法,而近年來CD24更被證實為巨噬細胞的免疫檢查點,從而開啟了新一代免疫檢查點療法新契機。我們團隊研發的治療性人源化CD24抗體HH0146,為國內人源化CD24抗體抗腫瘤專利技術的領先者,能專一性地活化巨噬細胞的優異吞噬腫瘤能力,同時也能激活自然殺手細胞以毒殺腫瘤,調節腫瘤免疫微環境,從而實現卓越的抗腫瘤效果。HH0146抗體藥已獲台灣發明專利及完成國際專利佈局申請(PCT/US24/13014:未公開)。
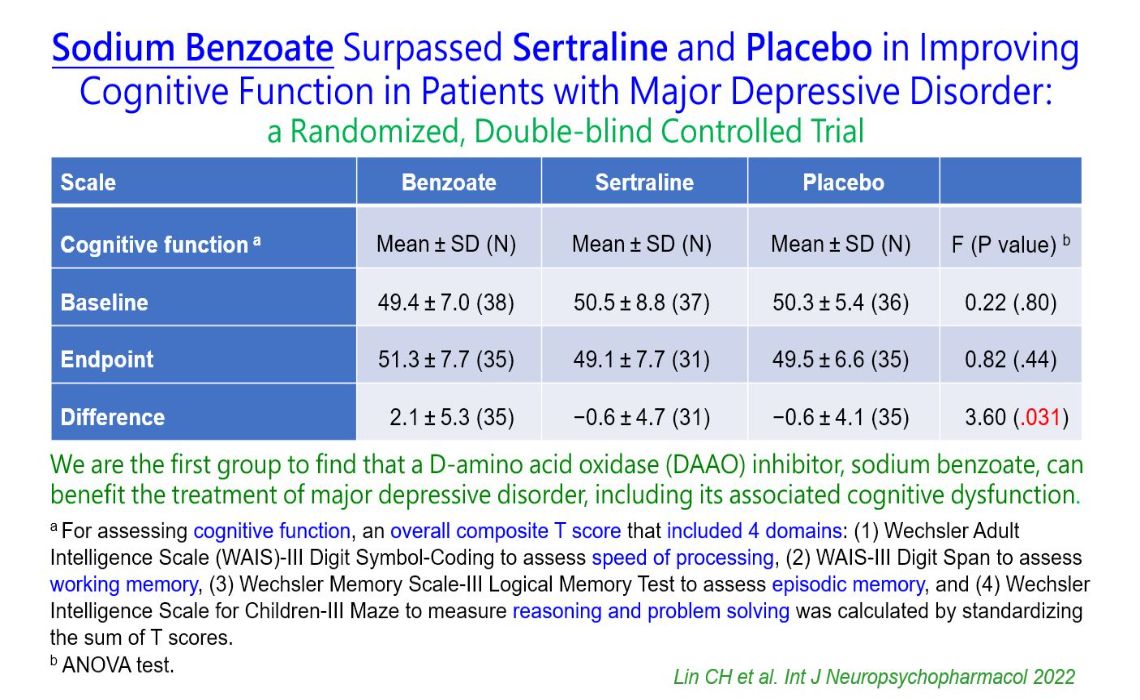
 右旋胺基酸氧化酵素抑制劑治療鬱症D-amino acid oxidase (DAAO) inhibitor for the treatment of depression高雄長庚紀念醫院精神部、中國醫藥大學生物醫學研究所Kaohsiung Chang Gung Memorial Hospital, China Medical University Graduate Institute of Biomedical Sciences林潔欣、藍先元Chieh-Hsin Lin, Hsien-Yuan Lane發明人:林潔欣、藍先元領域:新穎藥物/New Drug適應症:鬱症/Major depressive disorder研發階段:申請試驗用藥物/新醫材(試劑) Investigational New Drug (IND)/ Investigational Device Exemption (IDE) application摘要:鬱症是重大精神疾病。目前的抗憂鬱劑作用主要是建立在單胺理論,然而療效不佳、特別是對感知壓力與認知功能更是效果有限,並且有明顯的副作用。NMDA受體功能低下已被認為與鬱症的病理機轉有關。苯甲酸鈉是一種右旋胺基酸氧化酵素(DAAO)抑制劑,能提高右旋胺基酸的濃度,進而促進NMDA神經傳導。發明人經由兩項隨機雙盲臨床試驗發現苯甲酸鈉可以安全有效地改善鬱症患者的憂鬱症狀、感知壓力與認知功能,效果優於安慰劑與已上市抗鬱劑。本發明提出全新理論,係全球第一使用DAAO抑制劑治療鬱症,已獲得六國專利保護,可望發展為新一代鬱症治療藥物。
右旋胺基酸氧化酵素抑制劑治療鬱症D-amino acid oxidase (DAAO) inhibitor for the treatment of depression高雄長庚紀念醫院精神部、中國醫藥大學生物醫學研究所Kaohsiung Chang Gung Memorial Hospital, China Medical University Graduate Institute of Biomedical Sciences林潔欣、藍先元Chieh-Hsin Lin, Hsien-Yuan Lane發明人:林潔欣、藍先元領域:新穎藥物/New Drug適應症:鬱症/Major depressive disorder研發階段:申請試驗用藥物/新醫材(試劑) Investigational New Drug (IND)/ Investigational Device Exemption (IDE) application摘要:鬱症是重大精神疾病。目前的抗憂鬱劑作用主要是建立在單胺理論,然而療效不佳、特別是對感知壓力與認知功能更是效果有限,並且有明顯的副作用。NMDA受體功能低下已被認為與鬱症的病理機轉有關。苯甲酸鈉是一種右旋胺基酸氧化酵素(DAAO)抑制劑,能提高右旋胺基酸的濃度,進而促進NMDA神經傳導。發明人經由兩項隨機雙盲臨床試驗發現苯甲酸鈉可以安全有效地改善鬱症患者的憂鬱症狀、感知壓力與認知功能,效果優於安慰劑與已上市抗鬱劑。本發明提出全新理論,係全球第一使用DAAO抑制劑治療鬱症,已獲得六國專利保護,可望發展為新一代鬱症治療藥物。